Hope starts with the clinical trial
MCT
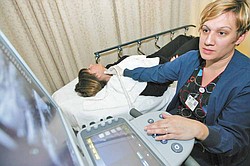
Sharon Gonzalez, Vascular Lab Technician at The Valley Hospital in Ridgewood, New Jersey, conducts a carotid ultrasound on Linda Pollack, Monday, January 24, 2011. Gonzalez used a Sonix Touch Ultrasound system and a grey scale imaging screen with zooming capabilities.
McClatchy Newspapers
HACKENSACK, N.J.
Is there a drug that can melt plaque off the walls of clogged arteries? Prolong the life of women with triple-negative breast cancer? How about a way to zap a tumor that is typically resistant to treatment?
These and other possible medical breakthroughs are among more than 500 clinical trials under way in hospitals in northern New Jersey alone.
The studies bring prestige and first-string specialists to hospitals, but more importantly, doctors say, they offer hope to patients.
“As an oncologist, if you have to walk into a room and tell a patient, ‘I’m sorry, there’s nothing more we can do,’ or have the chance to say, ‘We have another drug you can try,’ you certainly want to give them that option,” said Dr. Andrew Pecora, vice president of cancer services at the John Theurer Cancer Center at Hackensack, N.J. “With clinical trials, you’re offering tomorrow’s medicine today.”
Northern New Jersey hospitals, rich in diverse populations and top-shelf medical care, are prime for such trials. Most of the current studies focus on cancer, but dozens also involve cardiac disease. A handful target such illnesses as epilepsy, sickle cell, Alzheimer’s, lupus and sexual-arousal disorder in women.
“An institution like ours, with socioeconomic, cultural, ethnic and genetic diversity gives us the ability to study the broadest aspect of a disease and its treatment,” said Dr. Jai Parekh, an ophthalmologist and head of research for the Research Institute of St. Joseph’s Regional Medical Center. “A lot of strides have been made because of clinical trials and it gives our patients the ability to get the best treatment out there.”
One former Fair Lawn, N.J., resident is the recipient of such cutting-edge medical care.
Linda Pollack had a heart attack in December 2009 and had a stent implanted in a fully blocked artery. She was also given an unnamed statin for a partially clogged artery through a trial at The Valley Hospital in Ridgewood, N.J.
The drug worked; her latest angiogram showed an all-clear.
“It’s a miracle. I can’t believe it — the artery is opened all the way,” said Pollack, who has moved to Washingtonville, N.Y. “And you receive such great care when you’re in a trial. The doctors are always there for you. I was able to get an angiogram twice to see how I was doing. My insurance company wouldn’t approve an angiogram until I had my heart attack.”
Pollack was so pleased with her experience, she signed up for a second trial. This time, she may be taking either a placebo or what may be one of the most innovative drugs to reduce cholesterol.
This medication affects how the liver makes cholesterol, but it targets a “totally different enzyme system than a statin,” said Dr. Janet Strain, Valley’s director of cardiac clinical trials.
“We think this drug is going to be so effective it will melt the plaque on the arteries’ walls,” Strain said. “And we’re thinking that even if it doesn’t affect cholesterol that way, it may still reduce heart attacks or strokes.”
Most clinical trials don’t show such direct results. By design, many studies just observe how the body handles a particular drug, or if a certain dosage can be tolerated. Even in studies where patients are taking a drug for a disease, their lives are usually prolonged for only a few months.
“Trials don’t really benefit the participants — they benefit those who follow,” said Arthur Caplan, a professor of bioethics at University of Pennsylvania. “It’s usually a slow and difficult process.”
Trials, sometimes lasting for several years, are conducted after the federal Food and Drug Administration approves the studies.
“Clinical studies are necessary for medical research to continue — every device and drug out there has come into use because of clinical trials,” said Dr. Thomas Birch, medical director of the Institute for Clinical Research at Holy Name Medical Center. “We can’t continue to improve the treatments we offer without a considerable number of people in clinical trials.”
One drug being tested at both Valley and Englewood Hospital and Medical Center is expected to add five months to the life of women with triple negative breast cancer, a virulent disease.
“Preliminary data shows this is a promising drug,” said Cheryl Wild, manager of Valley’s oncology research department. “It inhibits the recurrence of cancer cells at the DNA level by stopping them from dividing.”
Another trial raising hope is on TrueBeam radiation treatment, a Hackensack study on tumors normally resistant to radiation. It zaps the tumor with radiation at four to five times greater than a normal dose, which may produce “a significant benefit,” Pecora said.
“Think of it as normally trying to put out a fire with a small amount of water — the cancer cells repair themselves before the radiation can kill them,” he said. “With this treatment, it would be like putting water on it at a rate that will put out the flames. It’s breathtaking.”
Copyright 2011 Associated Press. All rights reserved. This material may not be published, broadcast, rewritten, or redistributed.
 43
43